![]()
Chemical Reactions I: Net ionic equations
4.9A Natural Acid/Base Indicators: Red Cabbage Juice Extract
 Subjects: Chemical reactions - Acids/Bases
Subjects: Chemical reactions - Acids/Bases
Description:The relative pH of some foods and household chemicals is illustrated using red cabbage juice indicator.
Materials:
Dehydrated red cabbage juice
Scoop and plastic beaker
600 mL beaker
Water
8-10 100 mL beakers
0.1M aqueous HCl
0.1M aqueous NaOH
Any of the following: vinegar, ammonia, baking soda, lemon juice, milk of magnesia, clear soda, aspirin, vitamin C tablet, etc.
Optional: For fresh cabbage juice
4-5 leaves of fresh red cabbage
water
500 mL denatured alcohol
blender
funnel
cheesecloth or paper towel
600 mL beaker
‡Household items are located on their own shelf in the solutions storage cabinets.
*Additional glassware is located in the top drawer under the bin storage shelves and in the drawers to the right of the sink.
Pre-class Preparation:
1. Follow directions on the dehydrated cabbage juice instruction sheet for making the desired amount of indicator solution.
For fresh indicator:
1. Tear up the cabbage and load it into the blender.
2. Add the alcohol and water, and puree.
3. Strain the mixture to remove the solids using the cheesecloth and the funnel, collecting the extract in the large beaker. Dilute the extract with water.
Procedure:
1. Pour about 1 mL of the reconstituted indicator solution into the beakers with about 25 mL water.
2. Show first the color changes of the extract when a known acid (hydrochloric) and a known base (sodium hydroxide) are added.
3. Show the pH of any of the other materials
Discussion (Ref 1,2):
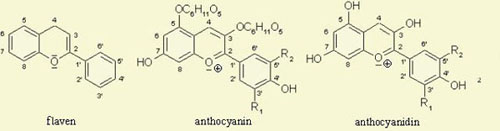
The purple color in the red cabbage is due to pigments called anthocyanins, a type of flavonoid compound. These same pigments are also responsible for the colors of many other flowers, fruits and vegetables. These are highly conjugated ring structures possessing a hydroxylated 2-phenylbenzopyrilium chromophore( 2) that can absorb light of certain frequencies, producing the colors we see. Acids and bases react with anthocyanins to produce anthocyanidins, which are resonance stabilized cations. See below (2):
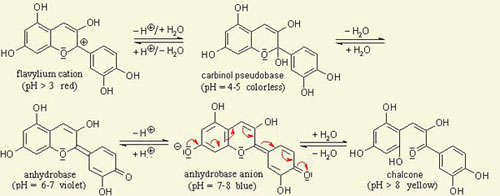
The color of anthocyanins is dependent on pH. The anthocyanidin system undergoes a variety of molecular transformations as the pH changes, producing various color changes. See below (2):
This demonstration also touches on the importance of chemical compounds in nature. You can discuss the extraction of useful chemical compounds (drugs (taxol), dyes, fragrances) from natural sources.
At the end, you can mix one of the acidic solutions with a basic one to try to get it back to purple.
Safety:
Use caution when handling some of the more caustic or corrosive of the household products, acids and bases.
Disposal:
Be sure that the solutions are within the proper pH range. These can be flushed down the drain with water. It is important to rinse out the cups very well before using them for the next demonstration or else you may be surprised by the colors you're getting! Throw the cabbage pulp in a wastebasket. Excess red cabbage juice can be flushed down the drain.
References:
1. Taken from: http://www.mit.edu/~chemistry/outreach/experiments.pdf
2. Images and text: http://www.demochem.de/p26_anth-e.htm


