![]()
Molecular Structure, Bonding, Orbital Hybridization
9.7 Polymerization - Barrel of Monkeys
 Subjects: Molecular structure, bonding, polymerization, organic molecules
Subjects: Molecular structure, bonding, polymerization, organic molecules
Description: A “Barrel of Monkeys” toy is used to demonstrate concepts in polymerization.
Materials:
- Barrel of Monkeys
- Plastic bottles, bags, etc as props
Procedure:
1. Take one monkey from the bin. Explain that the monkey represents a monomer.
2. Begin linking monkeys of the same color together. That chain represents a polymer. A polymer consisting of the same monomers is an example of a homopolymer. Just like the chain of monkeys is an example of a homopolymer of monkeys.
3. Place the monkeys back into their bin. Start with your monomer, but this time alternate colors. This is an example of a copolymer.
Discussion(ref 1):
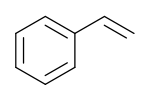
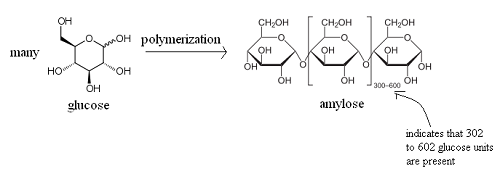
This simple demonstration illustrates the concept of polymerization. A single monkey represents a monomer – a molecule that can combine with others to form a polymer. Monomers are the smallest unit of a polymer. Some examples of monomers are given below. The second is glucose and the third is styrene.
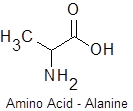
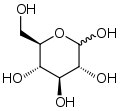
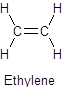
Several monkeys linked together represent a polymer – a large molecule composed of repeating units of monomers. Below is an example of the polymerization of glucose monomers to form the polymer amylose. Amylose is one of two components of starch.
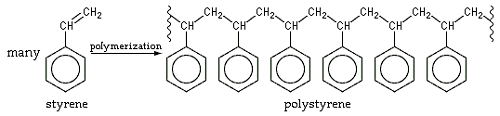
Polystyrene is the polymer that makes up Styrofoam. See the figure below.
Polyethylene is the most common polymer in the world. It is found in grocery bags, shampoo bottles, children’s toys, and even bullet proof vests.
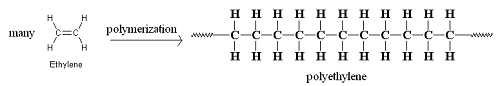
These are examples of homopolymers, which are polymers consisting of only one type of monomer.
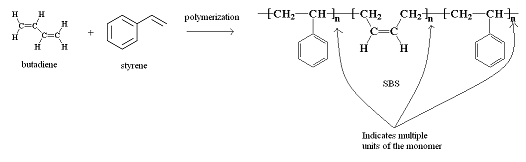
In contrast, a copolymer is a polymer that consists of more than one type of monomer linked together. Below is an example of a copolymer consisting of butadiene and styrene monomers to produce poly(styrene-butadiene-styrene) or SBS. SBS is a hard rubber used in the soles of shoes and tire treads.
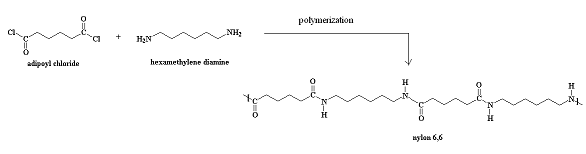
Nylon 6,6 is an alternating copolymer of adipoyl chloride and hexamethylene diamine. It is referred to as Nylon 6,6 because there are six carbons in the adipoyl chloride and six carbons in the hexamethylene diamine. Nylons are used in fabrics, thermoplastics, and in the case of nylon 6,6 it is used in nylon paint.
Safety:
None
Disposal:
None.
References:
1. Matt Huber, Jasmine Gilbert, F. Yarberry; Polymers. Word Document taken from
University of Central Arkansas STEM Institute:
http://uca.edu/stem/files/2011/07/Polymers.docx


