![]()
Atomic Structure, Periodic Trends
 (250x141).jpg) |
Use of props to demonstrate different types of electromagnetic radiation and atomic structure. |
| Display of atomic emission lamps containing different gases. Excitation of electrons in the gases result in emission of different wavelengths of light. |  |
 |
Alcoholic solutions of salts are combusted to illustrate that different wavelengths of light are emitted by different materials. |
| A beam of electrons is produced in a Crooke’s tube. A magnet is brought near one end of the tube and the electron beam is deflected into a bent line. |  (250x141).jpg) |
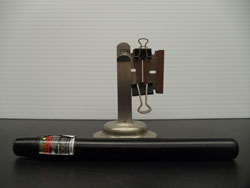 |
A beam from a laser pointer is directed through a narrow slit onto the far wall of the lecture room. An interference pattern can be seen on the wall. |
| Seemingly average materials, including rocks, are exposed to UV light, producing emission of various colors |
|
.jpg) |
A Geiger counter is used to detect radiation from certain objects. |
| A piece of granular calcium metal is added to water. After a few moments a vigorous reaction takes place producing hydrogen gas and calcium hydroxide. | .jpg) |


 (2).jpg)
